Bilcare Research Website Konzept und Kalkulation - PAGE-AND-PAPER_
LiveoOptima™
LiveoOptima™ is the first scientific method to determine the most cost effective blister packaging for solid dosage pharmaceutical products, following FDA recommended QbD principle. This patented technology determines the right barrier required to protect the product and maintain its efficacy. LiveoOptimaTM’s enhanced formulation analysis leads to the most cost effective barrier films to the requested shelf life period in all desired climatic conditions.
Discover how your product formulation can be scientifically matched to the optimal package with Liveo Research‘s scientific packaging services.


Science for better packaging
This process deduces the optimum packaging through a comprehensive drug sensitivity profiling, the output is not only a scientific background on packaging selection, but also a deep understanding of the product’s strengths and weaknesses. This sought after Liveo Research service has proven to provide up to 50% packaging cost reduction without compromising its quality and provided invaluable product knowledge to make faster, scientifically correct commercially beneficial decisions.
Traditional “trial and error” approach which wastes scientific time and resources will be replaced by commercial beneficial “right first time” solution while satisfying all regulatory and technical needs.
The LiveoOptima™ Methodology
- Degradation pattern of formulation is evaluated using a set of designed experiments
- Three batches are studied under various environmental conditions
- Effects of environmental variables on product critical stability attributes is assessed
- Estimates critical factors that determine failure mode and its threshold values
- Product sensitivity is quantified against each environmental factor
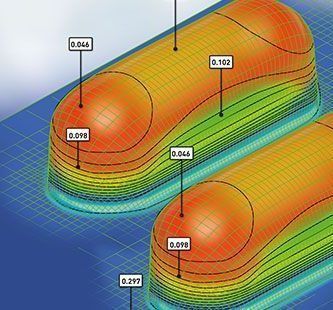
- Design the ideal blister cavity based on specific dose size and shape
- Simulate Cavity Thinning & Moisture Permeation through FEA Software to predict real-life MVTR
- Select the optimum blister film and foil lidding combination to get best shelf life

The Liveo +
- Outstanding customer service representatives
- Global and on-site application engineering support
- Local languages spoken by knowledgeable indigenous sales representatives
- Central point of contact for easy communication
- Low development costs
- Perfect product protection in respective climatic zone
- Avoidance of over packaging due to incorrect foil specification
- Reduction of waste, overpackaging and costs
- New film formulations developed in conjunction with customer input and requirements
Quality and Regulatory

-
Liveo Research pharmaceutical and medical packaging films comply with global applicable regulatory standards