Bilcare Research Website Konzept und Kalkulation - PAGE-AND-PAPER_
Liveo Modelling
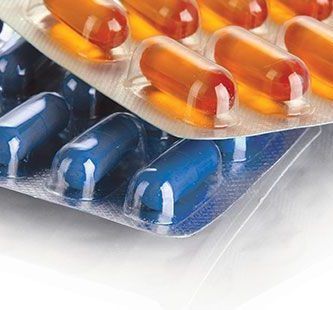
Most challenging aspect of blister packaging development is the difference in the barrier between the final blister and the barrier film that used to make the blister. Therefore the protection that a product gets in the final pack cannot be judged from barrier values specified in the film specification. In fact, this lack of information is the reason for many of the stability failures and wrong packaging selection.
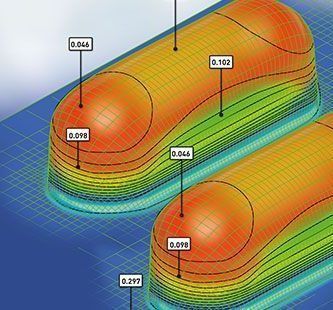
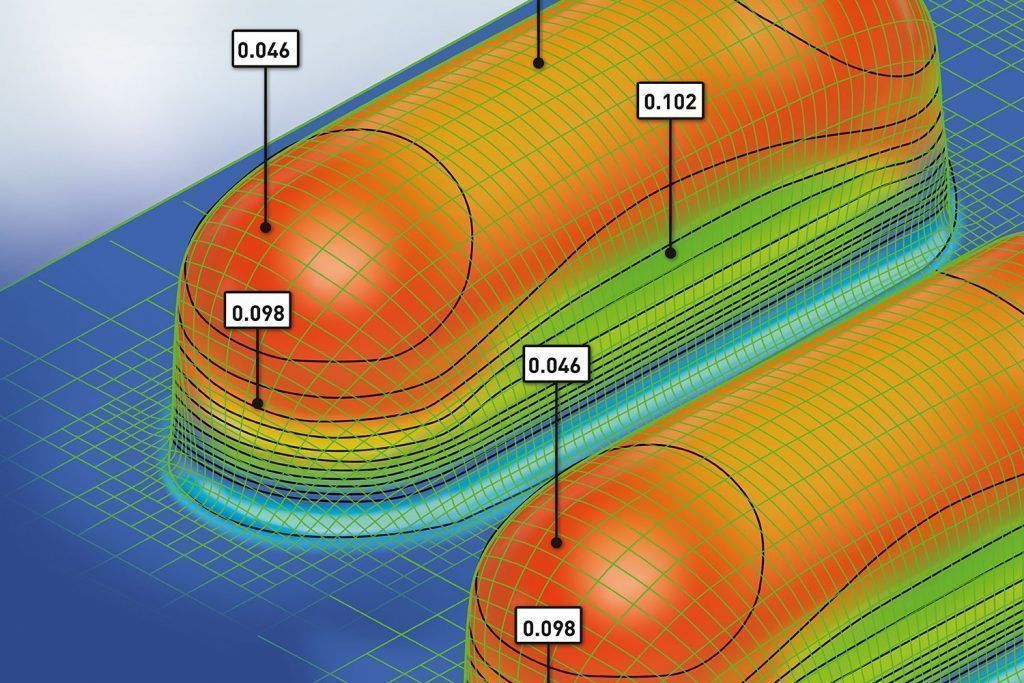
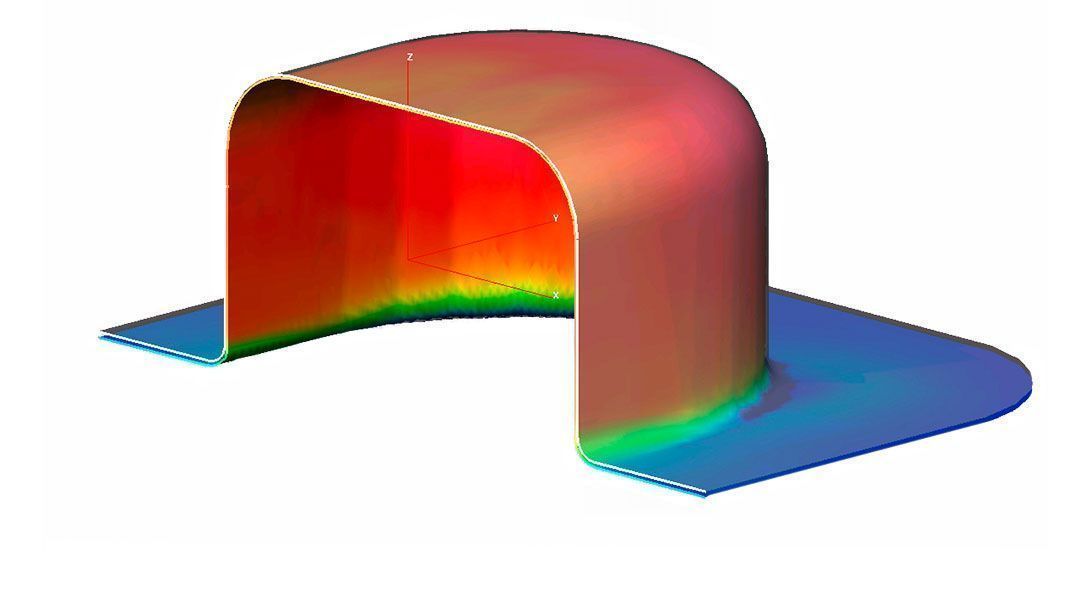
The barrier of any rigid thermoformable film is significantly influenced by film thickness. While forming the blister cavity the material thickness gets reduced and hence the barrier property changes. The thickness distribution is depending on the size and shape of the individual cavity. Therefore it is essential to know the thinning profile of materials after forming process and to determine the effective moisture barrier.
Do you have the ideal thickness distribution in your blister?
Let´s Check!
Solid and cost effective shape for solid dose products
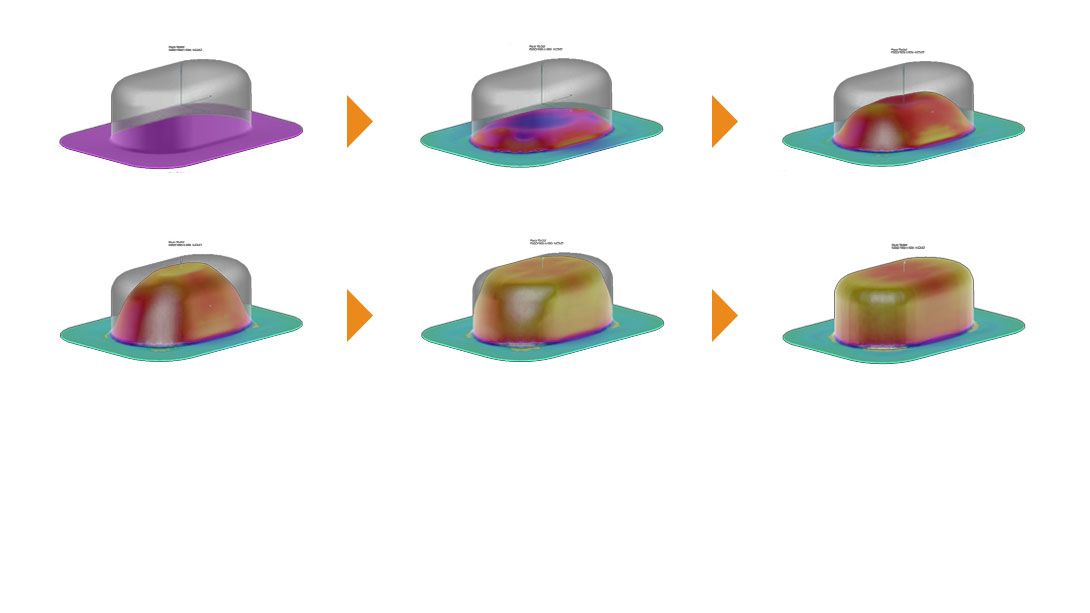
Liveo Research Modelling aids packaging engineers understand the thinning effects of the film after blister formation using a thermoforming simulation software based on Finite Element Method (FEM).
This is a customized adaption of a software tool used for many advanced applications. The results provide the guidelines for accurate material selection, cavity size, and forming process parameter setting. This allows package designers to explore the effects of tool geometry, film types, and process conditions before actually spending time on creating tools and before running actual experiments. Without physically producing blisters Liveo Engineers are able to identify the actual barrier properties (WVTR, OTR) of the virtual thermoformed cavity.
Solid and cost effective shape for solid dose products
- Predicts product and process feasibility during early product development
- Provides information on the barrier properties for the formed cavity
- Helps pharmaceutical manufacturers to quickly find a stability solution
- Reduces cost of product development
- Eliminates risk by identifying formability issues up-front through the graphical display of the forming zone, splitting, wrinkling and thinning
- Reduces the process development time by accurately sizing the cavities and improves material utilization
- Provides guidelines for accurate material selection, cavity size, and forming process parameter setting
The Liveo +
- Outstanding customer service representatives
- Global and on-site application engineering support
- Local languages spoken by knowledgeable indigenous sales representatives
- Central point of contact for easy communication
- Low development costs
- Perfect product protection in respective climatic zone
- Avoidance of over packaging due to incorrect foil specification
- Reduction of waste, overpackaging and costs
- New film formulations developed in conjunction with customer input and requirements
Quality and Regulatory

-
Liveo Research pharmaceutical and medical packaging films comply with global applicable regulatory standards